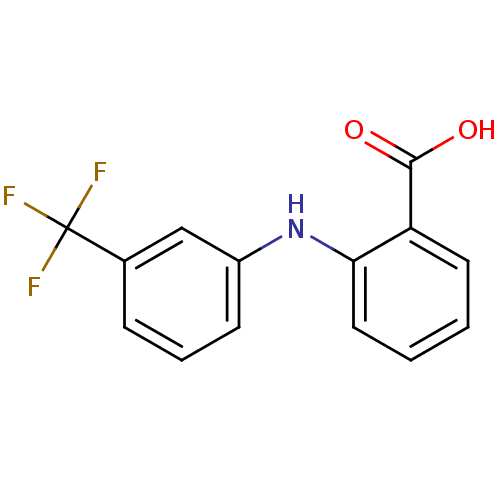
BDBM17636 2-{[3-(trifluoromethyl)phenyl]amino}benzoic acid::Arlef::CHEMBL23588::Flufenamic acid::Nichisedan::US20240002326, Compound Flufenamic acid::US9271961, Flufenamic Acid
SMILES OC(=O)c1ccccc1Nc1cccc(c1)C(F)(F)F
InChI Key InChIKey=LPEPZBJOKDYZAD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 17636
Found 8 hits for monomerid = 17636
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataIC50: 630nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataIC50: 370nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataIC50: 370nMAssay Description:Inhibition of AKR1C2 by fluorimetric methodMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataIC50: 3.14E+3nMAssay Description:Inhibition of human recombinant N-terminal His6-tagged AKR1C2 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataIC50: 3.14E+3nMAssay Description:Inhibition of AKR1C2 (unknown origin)More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataIC50: 370nMAssay Description:Inhibition of recombinant AKR1C2 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataIC50: 530nMAssay Description:Antagonistic activity against TP-receptor by inhibition of U 46619-induced contraction of isolated guinea pig tracheaMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataIC50: 530nMAssay Description:Inhibition of recombinant N-terminal GST-tagged human AKR1C2 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)